The 21 CFR part 11 / EU GMP Annex 11 regulations establish criteria for electronic records and electronic signatures to be trustworthy and reliable. The goal is to minimise the chances of data falsification and maximise the chances of detecting falsification.
To achieve this goal, SYNENTEC YT-AUDIT software uses advanced digital fingerprint technology and audit trailing in a highly automated manner for maximum compliance to 21 CFR part 11.

Streamlining 21 CFR part 11 Compliance with YT-AUDIT Imaging Software
The YT-Audit add-on uses advanced digital fingerprint technology and Audit trailing in a highly automated manner for maximum compliance to 21 CFR part 11.
Increasing efficiency not only leads to economic growth and optimal use of existing resources, it is also an essential component of competitiveness. Automation solutions are therefore more in demand than ever.
For example, digitised cell line development and production is also one of the guarantors of greater efficiency in the pharmaceutical industry. Here, the guideline of the U.S. Food and Drug Administration (FDA) 21 CFR Part 11, on “Electronic Records and Electronic Signatures,” enables the transition from paper-based manual processes to computer-supported electronic processes.
This requires working with 21 CFR part 11 compliant software, such as SYNENTECS’ newly developed YT-AUDIT Imaging and validation software.

More on SYNENTEC
SYNENTEC provide the most versatile cell imagers on the market. The instruments all have the option of full fluorescence capability. This means that the applications range from single cell cloning, apoptosis studies, through to transfection efficiency, toxicity studies and ultra-high throughput cell counting with viability. The instruments are compatible with a wide range of SBS format plate types and have a built in automation interface enabling integration into CLD platforms.
SYNENTEC’s team of engineers, software developers and biologists have developed automated, high throughput cell culture microscopes and fast, easy to use image analysis software.
The Cellavista, a high throughput fully automated cell imager, was launched in June 2007 and has been continuously developed ever since and was joined by the NyONE range of instruments in 2014. The instruments are used throughout the industry for applications varying from single cell cloning through to apoptosis studies.
Ellen’s take on this product
Versatile: Time saved by automation combined with precision optics and a wide range of applications make these imagers an integral part of any CLD workflow. Both extremely robust, the Cellavista offers higher throughput and is perfect for automated environments, while the smaller NyONE is ideal for medium-throughput laboratories with limited bench space. An extensive range of applications within Cell Line Development, Vaccine Research, Drug Discovery, Cancer Research, Stem Cell Studies and others makes these imagers the ultimate cell culture instrument.
Innovative: SYNENTEC are constantly developing new applications to meet the needs of customers. Technical assistance provided by the SYNENTEC team as well as local applications training means the user is supported from day 1.
Did you know?
For customers working in cGMP environments, SYNENTEC have developed YT AUDIT, a validation software which meets requirements for CFR21 part 11 compliance.
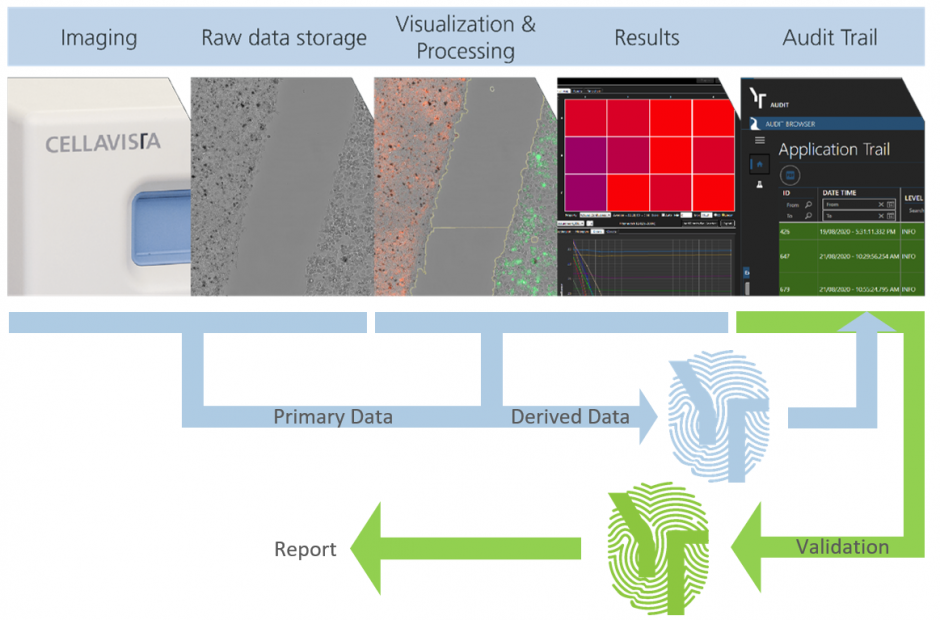
Highly automated 21 CFR part 11 compliant Imaging and Image Processing with YT-AUDIT
All GMP-relevant automated systems must be validated to ensure accurate, reliable and consistent data processing according to specifications.
In this context, every setting that can be changed in the software and has a direct influence on the result of the object to be measured is quality-relevant. Any change to this quality-relevant setting is recorded in the audit trail.
Settings without quality-relevant influence on the product need not and should not be included in the audit trail for reasons of overview and space requirements.
YT-AUDIT now takes care of all these decisions and control mechanisms for you. Fully automated.
The Benefits
- Protection of primary data (metadata & image raw data)
- Protection of derived data (processing and results)
- Audit browser for validation of experiments
- PDF report for experiments or templates of the audit trail
The three pillars of 21 CFR part 11 Compliance
- Group items
- Security management by customer‘s IT
- YTUser / YTManager / YTAuditor
- Certification by training
- Local database
- Digital fingerprinting technology
- Traceable validated experiment format
- Data integrity check
- Full traceability
- Multiple entries
- Searchable and filterable
- Exportable to PDF
Supports all common applications needed to run your R&D processes in high throughput
- Cell Line Development (e.g. Single Cell Cloning, CRISPR/Cas9 Tracking, Transfection Efficiency, Cell Viability Monitoring, PAIA Protein Titer Measurements, PAIA Glycosylation Measurements, Fluorescent Activated Single Cell Cloning (FASCC))
- Cancer Research and Drug Discovery (e.g. Imaging of 3D Spheroids, Toxicity Testing, IC50 studies, Cell Expansion Tracking, Apoptosis Monitoring, Nucleus Characterisation)
- Stem Cell Research (e.g. iPS Colony Count, Fluorescent Pluripotency Studies, Validation of Proliferation and Cell Migration, Cell Differentiation Analysis, Recombinant Lectin Probes)
- Immunology (e.g B-Cell and T-Cell Studies, Cytotoxic T-Lymphocyte Testing, Evaluation of Helper T-Cells and Subsets, Performing Cell Death Studies)
A user-friendly software interface will give you the choice of either using a predefined set-up from a list of established assays or to define your own work flow protocol and store this into a customised template.
Both imaging systems run on the same software platform which makes it easy to transfer your process in case of upgrading from NYONE for higher throughput of CELLAVISTA.