MTI Bioscience’s thermal sterilisers and can be used for applications ranging from media sterilisation through to viral inactivation. Flow rates range from 0.3L/min up to 12L/min. The systems are fully automated and can be customised to your exact requirements.
MTI Bioscience is a division of Microthermics who have been active in the food and beverage sector since 1989 where continuous processing and sterilisation is common place.
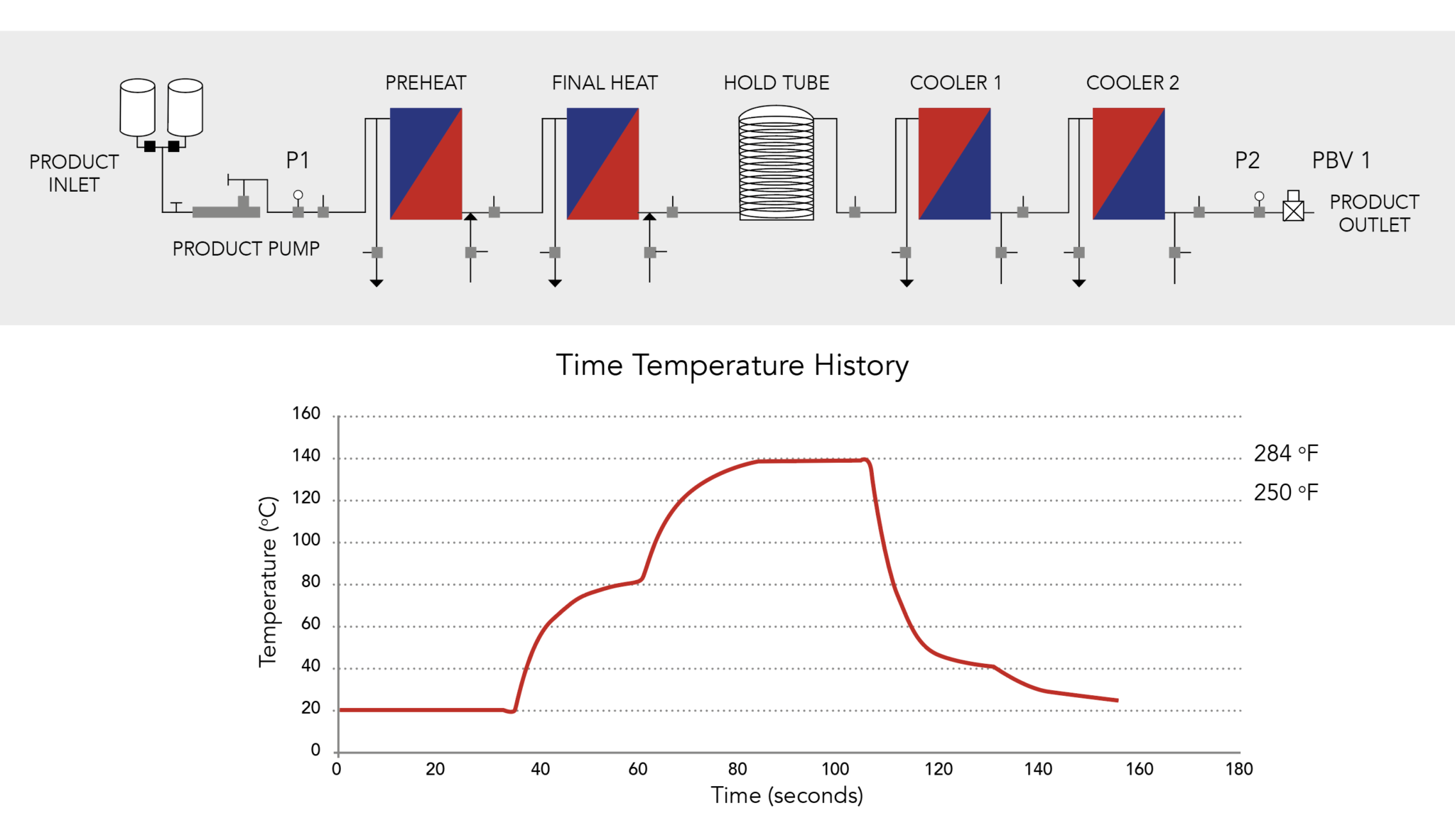
Continuous Thermal Process Flow-Diagram and Time-Temperature History
What is Continuous Thermal Sterilisation
In Continuous Thermal Sterilisation (CTS), media is pumped through:
- Heat exchangers to rapidly heat it to process temperatures of 138-150°C
- An insulated hold tube to retain it at temperature for just a few seconds
- Cooling heat exchangers to rapidly cool it to the required finish temperature
- The resulting overall heat exposure of the media is a fraction of what it sees in traditional autoclave processes.
- Additionally, the heat exposure is completely uniform.
Benefits
- Produces High Quality/Highly functional media with no degradation due to variable or prolonged heat exposure typical of autoclave and large volume bioreactors.
- Gentle process, can produce media failing in autoclave sterilisation and scale-up.
- Produces Completely Uniform Media o No variation within batch or from batch to batch like from autoclaving
- More energy, time and manpower efficient than autoclaving.
- Scalable – scaling to larger or smaller batches is quick and easy
- Real-time process validation to cGMP requirements due process control and stability
- Highly flexible configuration can easily switch from one product to another. Ideal for R&D and rapid production environments.
WHO TO CONTACT
Tom’s take on MTI Bioscience
Consistent: MTI’s continuous thermal sterilisers produce high quality and highly functional media with no degradation due to variable or prolonged heat exposure as can be the case with autoclaving and large volume bioreactors. The gentle process produces completely uniform media with no variation within batch or from batch to batch.
Scaleable: Continuous sterilisers are highly scaleable and are well suited to today’s move towards continuous processing. The amount of media produced is purely a function of flowrate and time unlike a batch process which can cause bottlenecks. MTI have a range of instruments available depending on the flowrate desired.
Did you know…
MTI’s entire range of instruments can be built to exacting cGMP standards as required by pharmaceutical production processes
Make an enquiry