APPLICATION NOTE by SECURECELL
Pharmaceuticals have a prominent role in healthcare and their manufacturing requires innovative tools, scientific and engineering knowledge, and must at the same time comply with strict regulations.
Efficient bioprocess development is therefore a challenging endeavour: timely monitoring and control of Critical Process Parameters (CPPs) and Critical Product Attributes (CPAs) are crucial to design, analyse, and control a bioprocess. The goal is not to have to test the quality of the product, but that it is built-in by design. Implementing Process Analytical Technology (PAT) and advanced data analytics is of paramount importance to comply with demands by regulatory bodies.
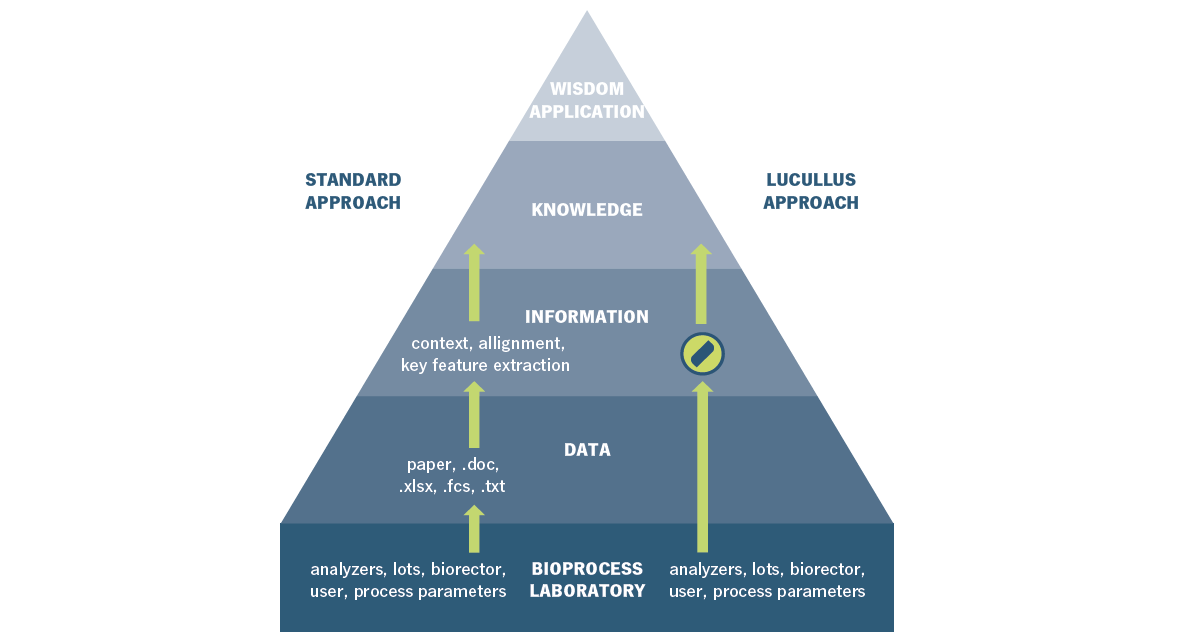
The above shows the practical approach from data to information to knowledge to wisdom or a final application. Illustration of the Lucullus® PIMS approach, which enables a significant speed-up in process development based on extensive data and information management.